Written by Patricia Daly
“Cancer as a metabolic disease” is a phrase coined by Dr. Thomas Seyfried.
Nutrition research is complex and has been subject historically to conflicting findings. This highlights the need to avoid taking headline-grabbing study outcomes at face value, instead opting for a more nuanced interpretation. It is not uncommon to find studies contradicting or reaffirming the exact same principles. This is an endless source of frustration, which we need to learn to navigate.
In This Article We Explore:
What do we mean by “cancer as a metabolic disease”?
The role of mitochondria
Why a ketogenic diet when facing cancer?
The many pathways targeted by the ketogenic diet
How a keto diet can impact many different aspects of life
Why is this approach not part of mainstream treatment?
Keto as the first port of call for cancer patients
At AWARE, we do not depend blindly on scientific evidence. Whilst our work is heavily informed by a scientific approach and we are certainly always up-to-date with the latest research insights, we also give weight to a person’s inner wisdom or “common sense”. Whilst this may involve a departure from strict scientific norms, decades of experience in nutrition have shown us that the body has an innate capacity to signal if a certain food, nutrient or drug is just not sitting right with it, no matter how many research papers have been published on its benefits.
As we constantly emphasise, the discussion surrounding a low-carb or ketogenic diet for cancer is at a critical juncture concerning science, and perhaps even politics. Current governmental guidelines, propagated by various organisations, including cancer societies, often adhere to the conventional “food pyramid” model. Yet, a growing cohort of individuals, including scientists and medical professionals, advocate for a paradigm shift. The tide is gradually changing, and we will elaborate on the reasons driving this transformation.
What do we mean by “cancer as a metabolic disease”?
A growing body of medical research has questioned the development of cancer and whether it truly has genetic origins. Based on what we have read in the media or what we have learned through the various schools of health and nutrition we follow, most of us tend to think of cancer as originating in the DNA of a cell when a series of mutations in a single cell happen.
This is the basis of the genetic explanation of cancer development, known as the “somatic mutation theory”, which has been the prevailing paradigm in cancer research over the past five decades. However, as this body of research has provided few outcomes or resolutions in terms of treating or preventing this disease, an increasing number of scientists have been looking for other approaches to cancer management. This alternative research has paved the way for a very important question: what if cancer has less to do with genes and more to do with the body’s metabolic processes? For clarification, metabolism refers to all chemical reactions that happen in a living organism to sustain life. In other words, it’s the way we use food, water, and oxygen to heal, grow or generate energy.
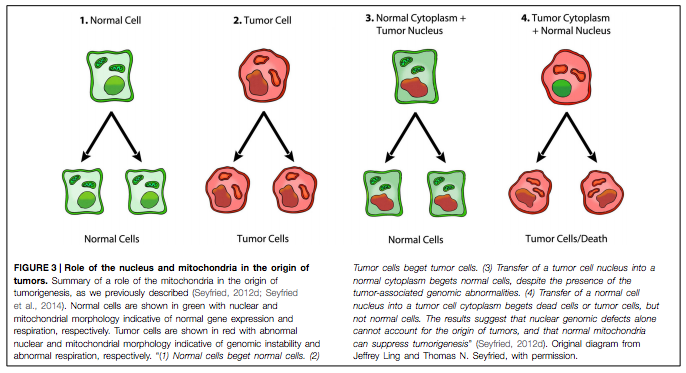
Professor Thomas Seyfried, residing at Boston College, is a renowned scientist whose pioneering research over the last few years brought to the fore many developments and insights in relation to “cancer as a metabolic disease”. He was particularly interested in so-called nuclear transfer experiments, as you can see in the graphic below:
“Normal”, healthy cells are shown in green with normal nuclear and mitochondrial functions. Tumour cells are seen in red with abnormal nuclear and mitochondrial morphology, indicating genomic instability and damaged respiration. On the very left, we see that if a healthy cell replicates, it begets two healthy daughter cells. When a cancer cell- with a cancerous nucleus and cytoplasm- divides, the result is two cancer cells. No big surprises there. Here’s where it gets interesting: when a tumour cell nucleus is transferred into a healthy cytoplasm with well-functioning mitochondria, cell replication begets two normal cells although there is genomic abnormality in the nucleus– which usually (according to the somatic theory) leads to cancer. Conversely, it was observed that the transfer of a healthy nucleus into a tumour cell cytoplasm (including abnormal mitochondrial function) resulted in tumour cells (or dead cells- via a process called apoptosis).
These findings were truly revolutionary because they are challenging how we have approached and understood cancer over the last few decades. At the core of this research is the idea that DNA mutations alone cannot explain the origins of tumours, while healthy mitochondrial function has the potential to suppress the formation of cancer cells. Although this idea may seem incomprehensible at first, this new research has shown us that one must consider it a very likely possibility- or at least a contributor- in the development of cancer.
The role of mitochondria
The idea that food alone may have the ability to profoundly affect your metabolism, may seem quite alien, or perhaps unrealistic. This is even more so if we claim that diet plays a fundamental role in preserving the health of your mitochondria and potentially protecting your cells from turning cancerous. This is where the ketogenic diet comes in.
Within the structure of a biological animal or human cell, the mitochondria are the small organelles filled with orange creases (called “cristae”).
Research has shown that the ways in which malignant cells make energy are very different compared to healthy cells. As the above graph by Prof Seyfried demonstrates, within diseased cells, there is usually some deficiency within the powerhouses of these cells, or the mitochondria, where 80-90% of the body’s total energy is produced. Indeed, many cancer cells have damaged, ineffective (and sometimes inexistent) mitochondria, which is one of the reasons why tumour cells rely on a very archaic, inefficient, and also “dirty” way of generating energy: glycolysis, or the splitting of glucose. This process happens in the cytoplasm outside the mitochondria and generates a lot less energy. As the energy requirements of a cancer cell are quite high, it compensates for this energy deficit by splitting glucose at a very high rate.
This mechanism is probably best demonstrated in PET scans, often used in the detection and staging of cancer. These scans provide a very important visual portrayal of glucose consumption in tissue when a patient is injected with radioactive glucose (e.g. FDG). This is a glucose analogue that is taken up very avidly by cancer cells that are aggressive, as opposed to healthy cells. This is due to a phenomenon called the Warburg effect. Otto Warburg, a famous biochemist, demonstrated 100 years ago that aggressive cancers may be hypoxic (not using oxygen) but that even if you expose them to normal oxygen conditions, they still retain this glucose and glycolytic dependence for energy (also called ATP in the cell) generation.
In simple terms, the more aggressive and potentially life-threatening a cancer is, the more it is going to light up on a PET scan, indicating that these malignant cells are out-competing healthy tissue for glucose availability. As such, PET scans have become the gold standard for diagnosing many cancer types (except for prostate cancer, for instance), allowing doctors to make a more accurate diagnosis by determining the location and aggressiveness of cancer cells.
What is the rationale behind a ketogenic diet for cancer?
It is already a well-established fact that many cancer cells are reliant on glucose. By reducing carbohydrates to about 3-10% of total calorie intake a day, the ultimate goal of a ketogenic diet is to keep blood sugar and insulin levels as low as possible. This alone will play an important role in weakening cancer cells.
Contrary to what many people believe, a ketogenic diet is not equated with a high protein diet. In fact, the keto approach dictates that protein intake should be at a moderate level for most people, and a low level in some cases. This is related primarily to the role of glutamine; an amino acid that has been shown in research (as well as other fermentable amino acids) to be used by certain cancer cells for growth and metastasis, almost as efficiently as glucose. As glutamine is a non-essential amino acid, meaning it can be produced by the body, there is no way of eliminating our exposure to it other than adjusting our diets. Moreover, there is currently no medical protocol or pharmaceutical drug that can do this.
Most people are also surprised to learn that protein can have an insulinogenic effect, which means it can trigger an insulin response. Not as much as carbohydrate-rich foods but in some cases, this needs to be taken into account. Insulin is a classic example of an anabolic hormone (used for growth and repair) and many cancer cells have a tendency to overexpress insulin and also IGF-1 receptors. In other words, insulin, which can be triggered by carbohydrate or protein-rich foods, may signal to cancer cells that the environment is optimal for growth and division. Importantly, high levels of insulin are not ideal during cancer treatment as they can have protective cancer properties, working to safeguard tumour cells. Research has shown that when insulin levels are low, cancer cells are more likely to be killed by radio or chemotherapy.
The many pathways targeted by the ketogenic diet
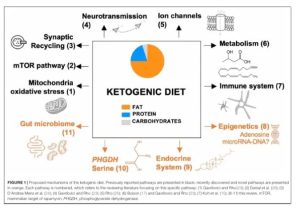
Importantly, despite the vast interest in the role of insulin, it isn’t the only pathway that is targeted by the ketogenic diet, which basically mimics a fasting state (more on this later!). Other important pathways in the cell like PI3 kinase, AKT, mTOR and AMP kinase are also affected positively for cancer patients, including those undergoing treatment. Here is an overview of some of the mechanisms and pathways- and, as everything in science, this is of course constantly evolving and
Source: The Ketogenic Diet Revisited: Beyond Ketones
Research has also shown that ketone bodies – the by-products of a ketogenic diet produced by the liver – have a positive influence on the epigenome, which means the way we express our genes. Moreover, ketone bodies also have a double-effect of inhibiting the growth of tumour cells, while simultaneously protecting healthy cells when they are exposed to stressful environments, such as during cancer treatment. It is no surprise that many companies within the health and wellness industry have sought to capitalise on the many proven benefits of ketone bodies through the production of ketone salts and esters. These products can help an individual go into ketosis quicker while eliminating some of the side effects.
According to Rainer Klement, a radiation oncologist from Germany, his first-hand experience with patients has shown that radiotherapy treatment can be more effective when ketone bodies are high. As such, he has discussed how there are studies underway that explore the possibility of cancer patients (or anyone following a keto diet) having a ketogenic shake in the morning, which will then permit a more relaxed approach to their diet for the remainder of the day. It is important to note that taking these so-called “exogenous ketones” (introduced to the body from an external source) will not have the same effect as when they are produced in the body endogenously by the liver as a result of dietary choices. Nevertheless, this is a development that must be closely monitored as such keto-inspired products could make the implementation of a ketogenic diet – and indeed treatment outcomes- more successful.
How quality of life can be affected
The impact of a keto diet for a cancer patient goes far beyond tumour management or slowed disease progression, as improvements may be felt in diverse areas of daily life and general wellbeing. Having worked with many patients who have implemented a keto diet, these are some of the things we have observed at AWARE Clinic:
- Improved weight management.
This could refer to losing, maintaining, or gaining weight, depending on the needs and personal journey of each individual. Once insulin is kept under control and digestion is optimised, the body is able to absorb the nutrients it needs from food more efficiently and reach its optimal weight, which is different for each individual.
- Better mood, focus and concentration; general improvement of neurological functions.
We have been astounded at the number of individuals who have reported experiencing better mood, focus, and concentration, which may be because the keto diet is much more satiating, meaning they no longer need to eat every two hours. As meal frequency reduces throughout the day, the body’s vital resources are not wasted on constantly digesting food, allowing for enhanced focus, a clearer mind, and more energy. Keto dieters also tend to experience better mental health, with fewer mood swings that are often associated with blood sugar imbalances. - Digestive health.
A substantial amount of research has proven that the gut microbiome (the mini eco-system in our digestive tract) plays a crucial role in our immune system, in mental health, and almost every system in the body. As such, the impact of the keto diet on the gut remains a very controversial subject, as many doctors and nutrition experts cast doubt on the fact that a high-fat diet can be beneficial for gut health. However, this point of view is a very misguided one that often stems from the lack of a proper understanding of the ketogenic diet and the different ways it can be tailored to suit the individual needs of each person. The main argument of these opposing doctors and health experts is that a lack of beneficial fibre (from not eating enough vegetables or whole grains) can be detrimental to gut health in the long term. Here at Aware we have substantial experience in applying the keto diet for many health conditions, and we have concluded that no one needs to stay ultra-low carb (i.e. 12g net carbs) for a very long time. Many other strategies can be used to help enhance ketosis without compromising on nutrients and fibre. In fact, we have found that most people can slowly start increasing their non-starchy vegetable intake once they are fully keto-adapted, thus gradually incorporating a larger amount of gut-friendly foods. However, the issue is not just quantity, as variety is key. We encourage those who come to us at Aware to consider a variety of colours and textures and to go beyond the conventional, such as eating the greens of leeks/spring onions, broccoli stalks, “chewy” leaves of cauliflower, sprouted seeds and beans, to name just a few. Incorporating such a varied and rich whole foods approach will most likely eliminate any worry about a lack of diversity in the gut flora. Of course, health is never a simple process or driven by a single factor but depends on many aspects that are unique to each individual. However, we can say with certainty based on our experiences that the more the body is used to burning fat, the more it takes to be moved out of ketosis. Importantly, keto-adaptation (i.e. teaching the body to burn fat efficiently) is a very dynamic process that needs to be monitored and adapted regularly. - Hormone balance.
Contrary to what you may have read on thyroid and other hormone imbalances, a keto diet can be great for a “hormone reset”. The key is in the proper implementation of the diet, always under the guided supervision of a qualified practitioner, and to make sure it is monitored regularly and that any unwanted side effects that last longer than a few days (e.g. sleep problems, feeling lethargic, digestive issues, menstrual irregularity) are adequately investigated. In fact, some practitioners use the ketogenic diet in the very short term (6-8 weeks) to rebalance and recalibrate the hormonal system.
- Recovery time after exercise
A well-tailored keto diet often makes the body more resilient, meaning it can bounce back incredibly well after exercise with less muscle ache, particularly after resistance and weight training. But it’s important to recognise that carbohydrate restriction for athletes- especially in combination with endurance training- will look very different to a therapeutic ketogenic approach! Athletes generally require more protein, can be more liberal with carbohydrates and use fat as a buffer to add calories. For competitions, metabolic flexibility is key- an athlete needs to be able to effortlessly switch between glucose and fat-burning modes to make energy! - Not being a slave to food
This is a biggie for most people and has a big impact on quality of life. As a keto diet is more satiating (if done right), eating becomes less frequent, and thus one learns to depend less on food, particularly overcoming emotional eating patterns, constant snacking, and food cravings. Furthermore, the body is better able to undergo prolonged fasts, which may be useful for practical reasons when food is not accessible, or for therapeutic purposes. - Feeling somewhat in control
Finally, it is crucial to acknowledge this factor: For those dealing with chronic illness, maintaining a sense of control over their future significantly impacts their mental and emotional well-being. Conventional treatments often pose challenges as individuals may feel powerless, merely following prescribed regimes without any personal input. This lack of autonomy can be difficult for those accustomed to questioning and actively participating in decisions about their lives. In such instances, adopting the ketogenic diet provides individuals with a tangible anchor. It empowers them to take initiative, measure effects, and monitor progress, offering a more participatory role in their health journey.
Why is this approach not more mainstream?
After reading the above and being presented with the scientific evidence that supports a keto approach, you may wonder why this is not part of conventional cancer treatment and an option offered to patients suffering from chronic conditions- not just cancer. Unfortunately, the ketogenic diet remains largely misunderstood, shrouded in many myths and misconceptions that have often made both patients and medical professionals wary of including it as part of any health protocol. For instance, many doctors and nutritionists have not yet fully grasped the difference between nutritional ketosis and ketoacidosis. Thankfully, this mindset is starting to gradually alter and we have observed some subtle changes like, for instance, Diabetes UK correcting the part of their website that states ketosis is a “dangerous state”.
It is undebatable that diabetic ketoacidosis is a very serious metabolic condition that can result in death as both glucose and ketone bodies rise to very high levels. In other words, it is unrestrained, involving excessive ketosis in the presence of excess glucose. Moreover, as a very high amount of the slightly acidic ketone bodies is released, they can quickly build up in the bloodstream and overwhelm the delicate acid-base buffering system of the blood. As a result, blood pH drops fast, and if no insulin is administered to slow the ketosis and fluids are replaced, this condition can be fatal.
It is vital to clarify, however, that if a patient can produce insulin- even if it’s very small amounts – it is near impossible to end up in ketoacidosis, except in exceptional circumstances, such as if we’re in very prolonged periods of starvation, engaged in prolonged severe exercise, or go on alcohol binges. Insulin regulates the utilisation of glucose for fuel in the cells, the flow of fatty acids, and the creation of ketone bodies, as we’ve seen earlier. Nutritional ketosis, however, is very different: it is a state of low blood glucose levels and- generally- slowly rising ketone bodies because the body takes a while to adapt. The only time acidosis can be a problem is if somebody goes into ketosis very quickly, although we have only ever observed this phenomenon in a child. However, the mother was instructed beforehand on possible complications, recognised the symptoms, and immediately took steps to remedy this problem. For this reason, as constantly stated, it is vital that if you choose to embrace a keto diet, it is done so under the supervision of an experienced health professional who knows the right blood tests to run before you embark on the diet and will be able to carefully monitor you!
Keto as the first port of call for cancer patients?
As the research is still inconclusive, it is not clear if we can ever reach a situation where we are 100% sure whether cancer is a metabolic disease or whether it’s of genetic origin. However, in cases where it is clear that cancer is indeed driven by metabolic imbalances, we firmly believe that a ketogenic diet should be the first port of call alongside conventional treatments. But of course, there are lots of factors influencing the rate and pace at which we go into ketosis. For instance, behaviours, goals, discipline, social circle, the environment, and others, play a crucial role in influencing how the keto diet takes shape for each person. Indeed, food is such a personal and emotional subject that it often can make the implementation of a ketogenic diet challenging or sometimes impossible. Furthermore, it is important to remember that there are many variations of a low-carb and ketogenic diet and if you’re determined to go this path, many options are waiting for you.




Recent Comments